In an era where safety and authenticity are paramount, the traceability of medicinal products has emerged as a crucial aspect of the pharmaceutical industry. This journey of ensuring safe and genuine products reaches the consumer is laden with both challenges and opportunities. As we delve into the realms of traceability, you and I will explore its significance, the hurdles encountered, and the potential that lies ahead.
Challenges in the Traceability of Medicinal Products

The endeavor to ensure the traceability of medicinal products is akin to navigating through a maze with various hurdles awaiting at every turn. While the end goal is rewarding, the journey is laden with challenges that demand attention and resolution. Let’s delve deeper into the intricacies of these challenges:
Technological Barriers
Adaptation: The pharmaceutical industry is continually evolving with the advent of new traceability technologies. However, adapting to these technologies is often a demanding task. The transition from conventional methods to sophisticated traceability systems requires a steep learning curve and a conducive infrastructure.
Integration: Incorporating new traceability systems with existing processes and systems can be a Herculean task. The compatibility of new technologies with the existing infrastructure is crucial for seamless operations.
Cost Factor
Investment: Implementing traceability systems demands a significant financial investment. The cost encompasses technology procurement, system integration, training personnel, and ongoing maintenance.
Return on Investment (ROI): The ROI may not be immediate and can be a concern for many stakeholders. However, the long-term benefits of ensuring safety and compliance are undeniable.
Regulatory Compliance
Ever-evolving Regulations: The regulatory landscape concerning the traceability of medicinal products is dynamic with new norms and standards emerging. Navigating through this myriad of regulations and ensuring compliance at every stage is a complex endeavor.
Global Disparities: Different regions have varying regulatory standards concerning pharmaceutical traceability. This disparity poses a challenge for global operations, necessitating a nuanced approach to comply with diverse regulations.
Data Management
Volume: The volume of data generated and required for traceability is enormous. Managing this plethora of information efficiently is paramount to ensure accuracy and compliance.
Privacy and Security: Ensuring the privacy and security of sensitive data is a pressing concern. Any breach or misuse of data can have severe ramifications.
Supply Chain Complexities
Multiple Stakeholders: The pharmaceutical supply chain involves numerous stakeholders, each with its set of protocols and interests. Achieving a cohesive traceability mechanism amidst this diversity is challenging.
Counterfeit Products: The menace of counterfeit products in the supply chain is a significant hurdle. Traceability systems must be robust to identify and weed out counterfeit products, ensuring only authentic medicines reach the consumers.
The challenges in the traceability of medicinal products are manifold, but not insurmountable. With collaborative efforts, technological advancements, and a proactive approach towards regulatory compliance, the pharmaceutical industry can stride over these hurdles towards a safer and more transparent supply chain.
Do you know? Role of Traceability in Sustainable Supply Chain Management
Opportunities in the Traceability of Medicinal Products
Despite the challenges, the traceability of medicinal products unveils a plethora of opportunities:
- Enhanced Safety: The primary goal, ensuring the safety of the consumer.
- Market Competitiveness: A traceability system can be a market differentiator.
- Consumer Trust: Building trust through transparency and authenticity.
The traceability of medicinal products is like opening a book where each page holds vital information about the journey of the product. As you turn the pages, you not only ensure the safety and authenticity of the product but also venture into a domain replete with opportunities. With the right blend of technology, regulatory compliance, and industry cooperation, the day isn’t far when the traceability of medicinal products will become a seamless aspect of the pharmaceutical industry, promising safety and trust in every pill you take.
Navigating through the intricacies of the traceability of medicinal products, and partnering with a seasoned tech-ally like Aeologic Technologies can be a game-changer. Utilizing our collaborative approach and cutting-edge tech solutions, we can significantly smoothen the path towards achieving robust traceability, ensuring every medicinal product’s journey is transparent, compliant, and secure from origin to consumer.
Also Read our trending blog: Traceability Solutions for Supply Chains with Examples
What is Traceability of Pharmaceutical Products?
The traceability of medicinal products encompasses the meticulous tracking of drugs from their origin through the supply chain till they reach the end consumer. This process ensures that the products remain authentic, safe, and in compliance with regulatory standards. Let’s see how!
Verification
Verification is the cornerstone of traceability, ensuring that each product is genuine and meets the stipulated quality standards. It’s like having a validating stamp at each checkpoint, confirming the product’s authenticity as it moves along the supply chain.
Documentation
Documentation is the narrative of the product’s journey. It provides a clear record of where the product has been, who handled it, and ensures that every phase adheres to the regulatory norms. It’s akin to having a detailed diary that narrates the tale of the product’s voyage through various stages.
Recall Management
Recall management is a crucial facet of traceability. In cases where a product is found to be faulty or unsafe, traceability systems enable swift and efficient recall, ensuring that the risks to consumers are mitigated. It’s the safety net that catches any anomalies, protecting both consumers and manufacturers from potential hazards.
Supply Chain Visibility
Having a clear sightline throughout the supply chain is fundamental in the traceability of medicinal products. It allows for real-time monitoring and verification of the products at each stage of their journey. This visibility is like having a transparent pathway that not only reassures the stakeholders but also paves the way for informed decision-making.
Regulatory Adherence
Traceability ensures that medicinal products comply with the stringent regulatory requirements set forth by health authorities. It’s like having a rulebook that each product follows diligently, showcasing a commitment to adherence and ensuring that only compliant products reach the marketplace. This adherence reinforces the industry’s dedication to maintaining a high standard of safety and efficacy in its offerings.
Do you know? Role of Traceability in Sustainable Supply Chain Management
What is Track and Trace of Pharmaceutical Products?
Track and trace, in the realm of pharmaceuticals, is like having a vigilant guard escorting the medicinal products from the manufacturer to the pharmacy shelves, ensuring its integrity at every checkpoint. It’s a more nuanced and detailed extension of traceability, aimed at ensuring the authenticity and safety of the pharmaceutical products at each juncture of its journey. As we delve deeper into this concept, you’ll find that it’s akin to having a protective shield around the medicinal products, safeguarding the product and the end consumer.
Real-Time Monitoring
One of the hallmark features of track and trace is real-time monitoring. Unlike traditional traceability which may focus on documentation at various stages, track and trace provide a continuous insight into the product’s journey. This real-time monitoring ensures that any discrepancy is immediately identified and rectified, thereby ensuring the authenticity and safety of the product.
- Immediate Alert Systems: Any deviation from the designated path or unauthorized access is immediately flagged, enabling swift corrective actions.
- Live Data Analysis: Continuous data flow helps in better decision-making and predicting potential issues before they escalate.
Authentication
Authentication is at the core of track and trace systems. It ensures that the product in question is genuine, unadulterated, and in compliance with the regulatory standards.
- Verification Codes: Unique codes assigned to each product help in verifying its authenticity.
- Anti-Counterfeit Measures: By validating the product at various stages, track and trace systems act as a robust barrier against counterfeit products entering the supply chain.
Compliance Assurance
The pharmaceutical industry is one of the most heavily regulated sectors, and adherence to these regulations is imperative. Track and trace systems play a pivotal role in ensuring compliance with the regulatory norms.
- Documentation: Detailed documentation of the product’s journey helps in demonstrating compliance with the regulatory standards.
- Audit Trails: The audit trails created by track and trace systems are invaluable during inspections and audits, ensuring a smoother compliance process.
Track and trace in medicine-making is not just a rule but a promise to keep people safe. It helps us through the tricky path of getting medicines from making to selling, making sure they are real, safe, and follow the rules. It shows the medicine industry’s promise to keep people healthy and trust in medicines.
Different Types of Product Traceability
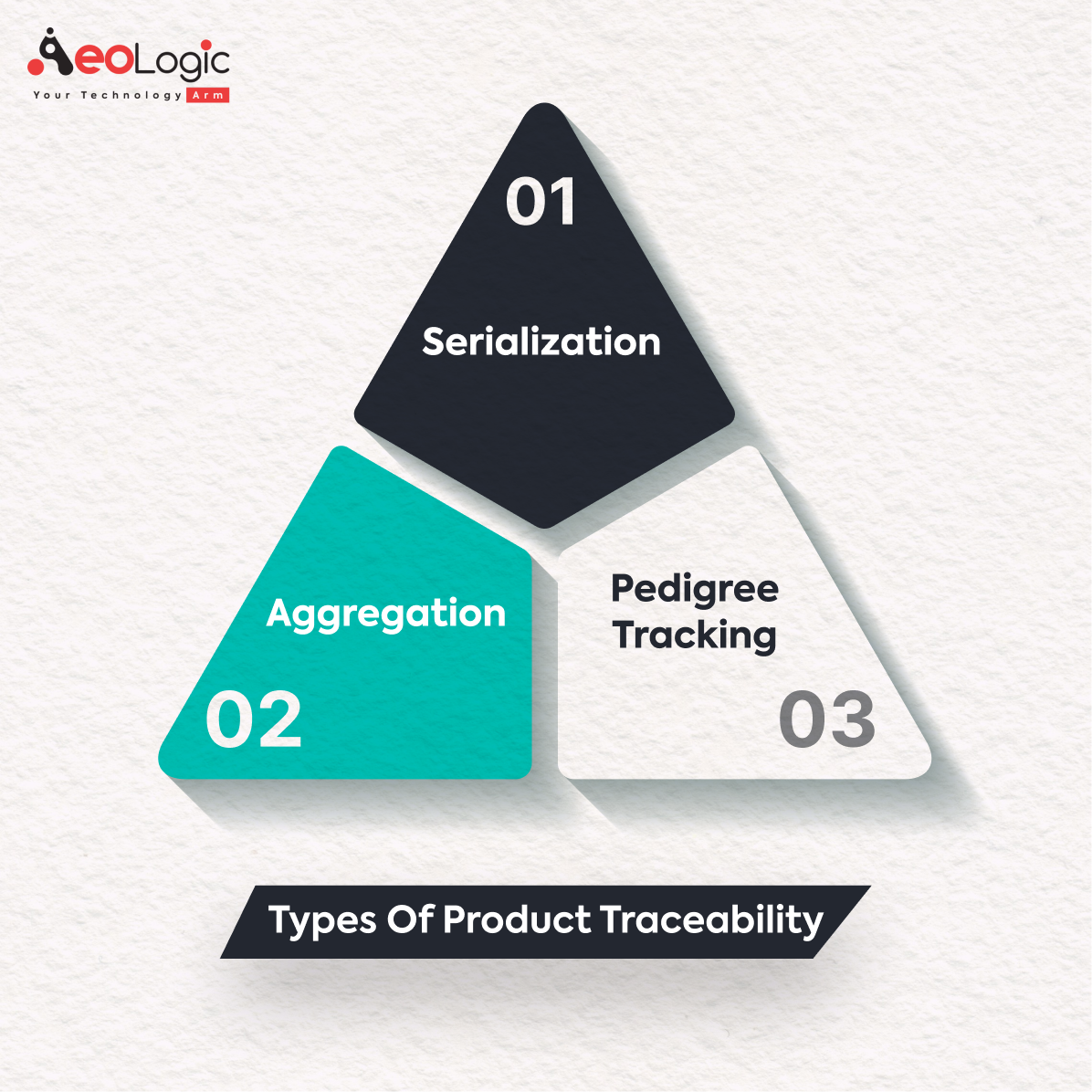
The different types of product traceability in the pharmaceutical domain are not just about adherence to regulatory mandates, but they signify a commitment to consumer safety and product authenticity. By integrating serialisation, aggregation, and pedigree tracking, a traceable network is created, fostering a culture of transparency and accountability. This trio of traceability not only navigates the complex channels of the pharmaceutical supply chain but also paves the way for enhanced consumer trust and market competitiveness.
The traceability of medicinal products can be achieved through various means, each with its unique attributes:
- Serialization: Assigning a unique identifier to each product, facilitating individual tracking and verification.
- Aggregation: Grouping of products and tracking them as a single unit, which simplifies the traceability process, especially during the packaging and shipping stages.
- Pedigree Tracking: A documented history of the product’s journey, ensuring a clear lineage of ownership and handling from manufacturing to dispensing.
By adopting a suitable combination of these traceability techniques, medicinal companies can significantly enhance the transparency and safety of their products, thereby elevating consumer trust and compliance with regulatory mandates.
Track and Trace Vs Traceability in the Medicinal Products
| Aspect | Track and Trace | Traceability |
|---|---|---|
| Focus | Real-time monitoring | Documentation |
| Scope | Detailed, at each stage | Broader, overall journey |
| Benefit | Immediate authentication | Efficient recall management |
| Technology Dependency | High, requires sophisticated systems | Moderate, basic systems can suffice |
| Cost | Higher due to advanced tech requirements | Relatively lower |
| Data Volume | Massive, due to detailed tracking | Less, due to broader tracking |
| Implementation Complexity | More complex, needs rigorous training | Less complex, easier adaptation |
| Regulatory Compliance | Strict, due to real-time monitoring | May vary, based on documentation |
| Consumer Insight | Provides detailed insight into product journey | Provides general insight into product journey |
Final Words
Embarking on the traceability journey within the pharmaceutical realm is more than regulatory compliance, it’s a pledge towards safety and authenticity. As we step into an era where transparency is a virtue, the traceability of medicinal products stands as a beacon of trust and assurance for both, the industry and the consumers.