Introduction
In the medical device assiduity, traceability is essential for making sure of the patient safety, compliance with regulations, and minimizing threat. Medical device distributors play a critical part in ensuring that traceability is maintained throughout the supply chain, from the manufacturer to the end user. In this blog, we will discuss why is traceability essential for medical device distributors and the benefits of enforcing an effective traceability system.
Traceability essential for medical device distributors refers to the capability to track and trace the movement of medical devices throughout the supply chain. This includes tracking the origin and destination of devices, as well as any intermediate stages in the distribution process. Effective traceability requires the use of systems and technologies that can capture, store, and manage data about the devices, including product information, periodical figures, lot figures, and expiration dates.
Also read: What do Traceability Solutions Mean to Your Business?
Why is Traceability Essential for Medical Device Distributors?
There are several reasons for why is traceability essential for medical device distributors as it is critical to the success of the medical industry.
First, traceability is necessary for compliance with regulations. The medical device assiduity is largely regulated, and distributors must deal with a variety of regulations and norms related to traceability. For illustration, the US Food and Drug Administration (FDA) requires that medical device distributors maintain records of all devices they distribute, including lot figures, periodical figures, and other relating information. In addition, distributors must be suitable to give this information upon request to the FDA or other supervisory bodies. Failure to deal with these regulations can affect in fines, penalties, and damage to a distributor’s character.
Second, effective traceability is critical for ensuring patient safety. Medical devices play a critical part in patient care, and any faults or defects in these devices can have serious consequences. Traceability can help identify and insulate defective devices, track devices that have been recalled, and help devices that have expired from being used. This can help prevent patient detriment and ameliorate the quality of care.
Third, traceability can help distributors minimize threat and ameliorate effectiveness. By tracking the movement of devices throughout the supply chain, distributors can identify possible backups or areas for enhancement. They can also quickly identify and address any issues that arise, similar as shipping delay or supply shortages. This can help minimize the threat of stock outs or other supply chain dislocations that can impact business operations and profitability.
Importance of Traceability
Eventually, traceability can give precious perceptivity into business performance. By collecting and assaying data about device movement and operation, distributors can gain a better understanding of client requirements and preferences. They can also identify openings to ameliorate effectiveness and profitability, similar as by optimizing supply operation or streamlining logistics processes.
What is Needed for Effective Traceability?
Enforcing an effective traceability system requires a combination of technology, processes, and people. Distributors need to invest in systems and technologies that can capture, store, and manage data about medical devices. This includes supply operation software, barcoding and scanning systems, and data analytics tools. They also need to establish clear processes and procedures for capturing and sharing data. For example, standardizing lot and periodical figures and enforcing a system for tracking device movement. (https://www.sullivansusa.net/) Eventually, they need to insure that their staff is duly trained and equipped to use these systems and processes effectively.
Moreover, traceability is essential for medical device distributors who want to insure compliance with regulations, ameliorate patient safety, minimize threat, and optimize business performance. Enforcing an effective traceability system requires a significant investment of time, team, and resources. But the benefits can be significant. However, by investing in traceability, medical device distributors can ameliorate their operations, enhance their character. And eventually give better care to patients.
Leveraging Traceability for Optimal Efficiency
Traceability offers a range of features that optimize effectiveness for medical device distributors. Some of them are:
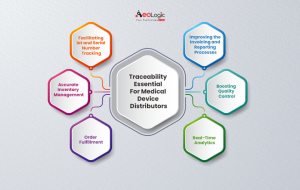
- It facilitates lot and periodical number tracking, ensuring accurate supply operation and effective order fulfillment. Distributors can rapidly identify and detect specific devices or batches, reducing response times and perfecting client satisfaction.
- Enables distributors to automate order processing, invoicing, and reporting processes. This automation helps in saving time, reducing manual mistakes, and improving functional effectiveness.
- Real-time analytics and reporting capabilities allow distributors to gain precious perceptivity into their operations, identify backups, and make data-driven opinions. In order to ameliorate effectiveness and productivity.
- Integrates with quality operation systems, enabling distributors to track and validate quality control processes throughout the supply chain. By automating quality control workflows and attestation. Distributors can insure harmonious product quality and alleviate the threat of non-compliance with assiduity regulations.
Also read: The Importance of Traceability in Supply Chain Management
Conclusion
In an decreasingly regulated and competitive medical device assiduity, distributors need robust traceability results to insure effective operations and compliance with regulatory conditions. Traceability offers medical device distributors a comprehensive tool set that enables end- to- end visibility, automates processes and enhances functional effectiveness. Also, by using the power of Traceability solutions, distributors can streamline their operations, ameliorate product safety. And eventually deliver better healthcare issues to cases. Furthermore, embracing this technology isn’t just a matter of staying ahead in the assiduity. But also fulfilling the pivotal responsibility of furnishing safe. And dependable medical devices to healthcare professionals and cases likewise. For complete information on Traceability Solutions, you can communicate with our industry experts.